Now that all individuals age 16 or over are eligible to be vaccinated in Los Angeles County, Cal State LA students share their vaccination experiences and pending questions.
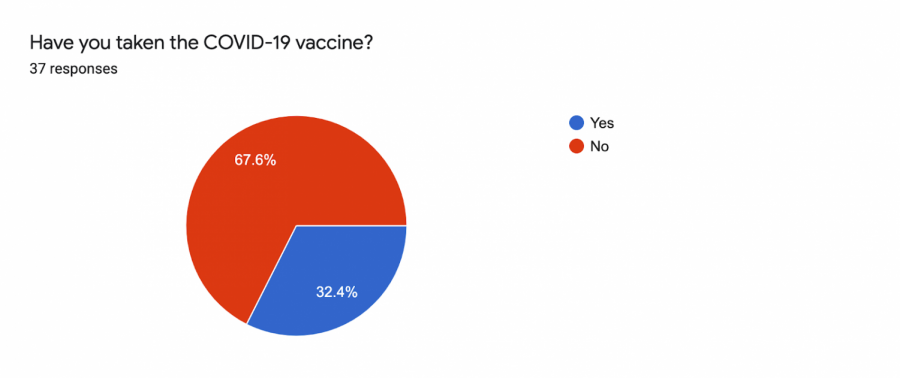
In an informal survey with 37 student responses conducted by the University Times (UT), only 32.4% of students have been able to take the vaccine.
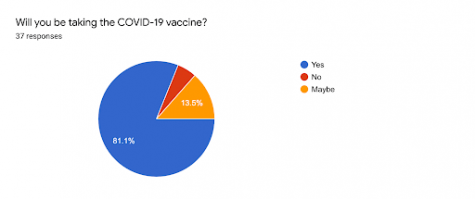
The survey also showed that 81.8% of students will be getting a COVID-19 vaccine compared to 13.5% of students who are still debating their decision.
Maribel Martinez, a 2020 grad student, got her final vaccine dose of Pfizer at the Cal State LA vaccination site. Martinez said she decided to get vaccinated because she and her family contracted COVID-19 back in December 2020.
Although she took precautions to prevent being infected with the virus, Martinez contracted the virus from a family member who was an essential worker.
“People think it is just the flu and they will not stay home or believe it until it hits close to their home,” said Martinez.
After taking her first dose, Martinez said she felt soreness and swelling in her arm along with some “COVID symptoms” like headaches, chills, body aches, and a runny nose. She added that this scared her because she remembered what it was like to have COVID-19. However, this time she said she slept a lot and felt better in 72 hours.
According to the Centers for Disease Control and Prevention (CDC), side effects like these are normal signs that the body is building protection and they should go away within a few days. Other side effects can include the symptoms that Martinez experienced, in addition to fever, nausea and tiredness.
Martinez said that the best way to fight this virus is by getting vaccinated since there are people out there with chronic illnesses like her husband.
Vanessa Vargas, a child development major, got her first dose of Moderna in early March. Vargas decided to get the vaccine when she contracted COVID-19 in January 2021.
Vargas said she felt drowsy and sleepy after getting her first dose along with other mild side effects that were reminiscent of a cold. Vargas added that the side effects were similar to COVID-19 symptoms she experienced but were not as harsh.
However, Vargas said she would like to know how long the vaccine will be in her body in order to protect her from contracting COVID-19 again. According to a U.S News article, Pfizer’s ongoing trial indicates the company’s two-dose vaccine remains highly effective for at least six months, and likely longer. People who got Moderna’s vaccine also had notable levels of virus-fighting antibodies six months after the second required shot.
Communication major Arie Lea said she is fully vaccinated with the Moderna vaccine. As a student who has asthma and works with a special needs child, things would be complicated if she contracted COVID-19. She added that she doesn’t want to contract the virus and end up giving it to the child she works with, who is only 10-years-old.
Lea said she experienced soreness in her arm after the first dose and body aches, chills, and tiredness after her second dose. She added that she wishes there was more research and data on how long the immunity will last.
As soon as he found out about his eligibility to get the vaccine, Cal State LA professor and curator for a coronavirus resource center William London got his first dose of the Moderna vaccine on March 2.
London said that after doing research, he found in the large rigorous clinical trial that all three vaccines are very safe and work extremely well at preventing severe coronavirus disease. After his first dose, London said he only felt soreness in his arm.
“We need to encourage many hesitant people to get vaccinated,” said London. His main concerns involve making vaccinations more available to underserved communities.
In the survey conducted by the UT, students were also encouraged to share any concerns or questions that lingered in their minds about the vaccines.
Students questioned the safeness of the vaccines, how to find out their eligibility and how accessible the vaccines are.
According to the CDC, the COVID-19 vaccines are safe and effective. The vaccines themselves were evaluated in tens of thousands of participants in clinical trials. They met the U.S Food and Drug Administration’s (FDA) rigorous scientific standards for safety, effectiveness, and manufacturing quality needed to suppose emergency use authorization (EUA).
The county’s health department website offers information on what type of vaccine you should get based on your age, checking your eligibility or making an appointment for a vaccine. Other resources are available online through websites like MyTurn.CA.gov.
The county’s health department also offers guidance on how to prepare before and after your appointment and provides resources to those with disabilities or without computer access. For assistance with appointments, you can call at 833-540-0473 between 8:00 a.m. and 8:30 p.m. seven days a week.